Unleashing the Power of Baking Soda and Sulfuric Acid Reactions

Ever wondered what happens when the common kitchen staple baking soda meets the potent industrial chemical sulfuric acid? This seemingly simple encounter unleashes a fascinating chemical reaction with various applications, from classroom demonstrations to industrial processes. Let's delve into the intriguing world of this chemical interaction and uncover its secrets.
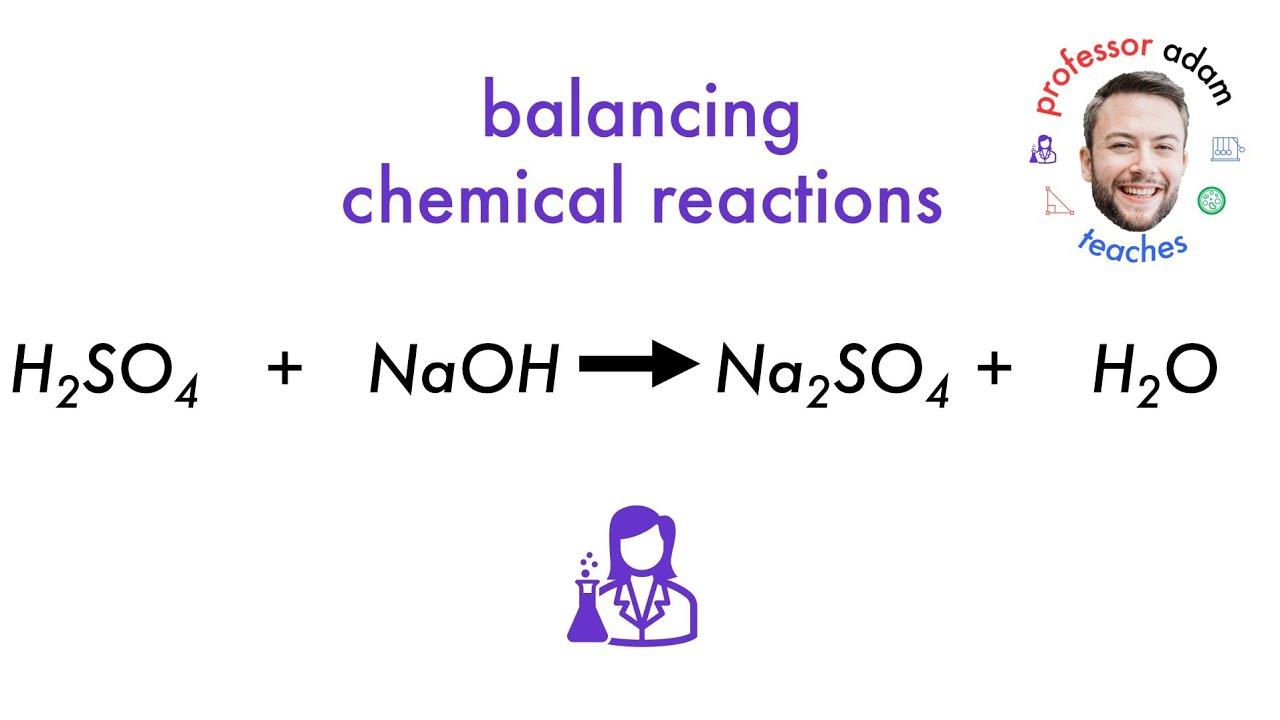
The reaction between baking soda (sodium bicarbonate) and sulfuric acid is an acid-base reaction, a fundamental concept in chemistry. When these two substances come into contact, they engage in a vigorous exchange, producing sodium sulfate, water, and carbon dioxide gas. This effervescent reaction is visually captivating, often marked by bubbling and foaming as carbon dioxide is released.
The interaction of sodium bicarbonate with sulfuric acid has a rich history, intertwined with the development of modern chemistry. Early chemists recognized the distinct properties of these substances and explored their interactions. Understanding this reaction played a crucial role in advancing our knowledge of acid-base chemistry and paved the way for countless practical applications.
The importance of this chemical interaction extends beyond theoretical understanding. It finds applications in diverse fields. For instance, the release of carbon dioxide can be harnessed in baking, contributing to the rise of cakes and bread. Furthermore, the reaction can be used in laboratory settings for generating carbon dioxide gas for various experiments.
However, it's crucial to acknowledge the potential hazards associated with the combination of sodium bicarbonate and sulfuric acid. Sulfuric acid is a strong acid and can cause severe burns if mishandled. The reaction itself can be quite vigorous, potentially leading to splashes and spills. Therefore, appropriate safety precautions are paramount when working with these chemicals. Always wear protective eyewear and gloves, and conduct the reaction in a well-ventilated area.
The chemical equation for this reaction is: 2NaHCO3 + H2SO4 → Na2SO4 + 2H2O + 2CO2. This equation clearly illustrates the reactants and the products formed.
One simple example of this reaction is adding baking soda to a solution of sulfuric acid. The resulting bubbling and fizzing demonstrates the release of carbon dioxide gas.
While the reaction has limitations in large-scale industrial applications due to cost and alternative methods, its educational value is significant. It's a common demonstration in chemistry classes, effectively illustrating acid-base reactions and gas evolution.
Advantages and Disadvantages of Baking Soda and Sulfuric Acid Reaction
| Advantages | Disadvantages |
|---|---|
| Visually engaging demonstration of acid-base reaction | Sulfuric acid is corrosive and requires careful handling |
| Useful for small-scale CO2 production | Reaction can be vigorous and potentially hazardous |
| Educational tool for chemistry concepts | Limited large-scale industrial applications |
Frequently Asked Questions:
1. What is the chemical formula for baking soda? (NaHCO3)
2. What type of reaction occurs between baking soda and sulfuric acid? (Acid-base reaction)
3. What gas is produced? (Carbon dioxide)
4. Is sulfuric acid dangerous? (Yes, it is corrosive)
5. What precautions should be taken when performing this reaction? (Wear safety goggles and gloves, work in a well-ventilated area)
6. What are some practical applications of this reaction? (Baking, laboratory experiments)
7. What is the chemical formula for sulfuric acid? (H2SO4)
8. What is the product besides water and carbon dioxide? (Sodium sulfate)
Tips and Tricks: Always add the acid to the baking soda slowly to control the reaction rate.
In conclusion, the reaction between baking soda and sulfuric acid is a fundamental chemical process with diverse applications. Its significance lies not only in its practical uses but also in its educational value. While the reaction presents potential hazards if mishandled, appropriate safety measures ensure a safe and insightful exploration of this fascinating chemical interaction. Understanding the intricacies of this reaction opens a window into the broader world of acid-base chemistry and underscores the importance of safety in scientific exploration. By studying these interactions, we gain valuable insights into the fundamental principles governing our world. Continue exploring, continue learning, and always prioritize safety.
Unlocking time navigating opm title 5 compensatory time off
Soothing shades exploring popular blue gray paint colors
Remembering mary ann holley a life celebrated