The Alchemy of Balance: Unveiling the Products of Neutralization
Imagine a perfectly tailored suit, the precise balance of form and function, a harmony of elements coming together to create something both beautiful and practical. In the world of chemistry, neutralization reactions achieve a similar equilibrium, a delicate dance between acids and bases that results in a transformative outcome. But what exactly does neutralization produce? This seemingly simple question unlocks a world of understanding about the fundamental principles governing our physical world, from the mundane to the extraordinary.
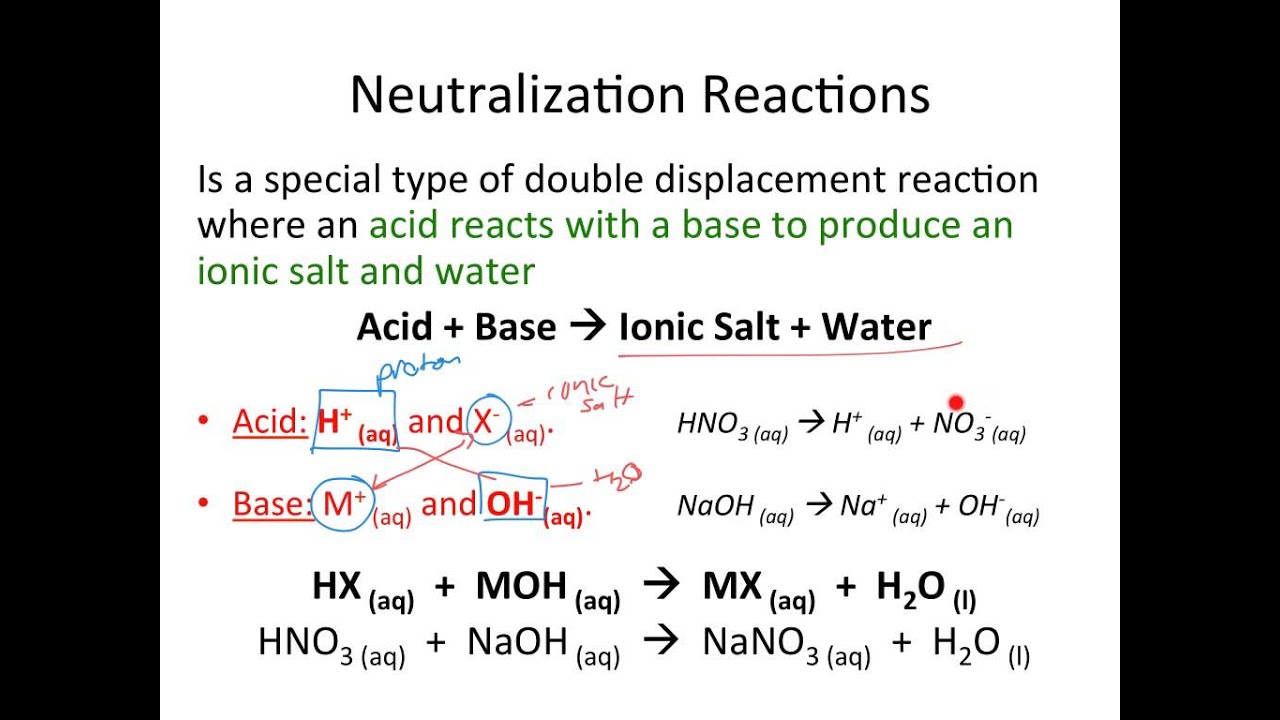
Neutralization, at its core, is the interaction between an acid and a base, resulting in the formation of salt and water. This reaction, often depicted with elegant chemical equations, represents a fundamental concept in chemistry. Think of it as a balancing act, where the acidic and basic properties cancel each other out, producing a neutral solution. The products of this reaction, salt and water, are essential components of life and play crucial roles in various natural processes and industrial applications.
The history of understanding neutralization dates back centuries, with early alchemists observing the interaction between acidic and basic substances. Over time, scientists like Antoine Lavoisier and Svante Arrhenius contributed to our understanding of acids, bases, and the nature of neutralization. The development of the pH scale provided a quantitative measure of acidity and alkalinity, further refining our comprehension of these reactions. The importance of neutralization extends far beyond the laboratory. It plays a vital role in environmental science, regulating the pH of soil and water, and in human physiology, where it helps maintain the delicate balance within our bodies.
One of the main issues surrounding neutralization reactions is the potential for uncontrolled reactions and the generation of excessive heat. Understanding the stoichiometry of the reaction, the precise ratio of reactants required for complete neutralization, is crucial for controlling these reactions and ensuring safety. The improper disposal of neutralized solutions can also pose environmental concerns, highlighting the importance of responsible chemical handling and waste management.
Let's explore a simple example. When hydrochloric acid (HCl), a common stomach acid, reacts with sodium hydroxide (NaOH), a strong base, it produces sodium chloride (NaCl), common table salt, and water (H2O). This simple reaction illustrates the transformative power of neutralization, converting corrosive substances into harmless compounds.
A benefit of neutralization is its role in wastewater treatment. By carefully adjusting the pH of wastewater through neutralization, harmful pollutants can be precipitated out, making the water safe for discharge or reuse. Neutralization is also essential in agriculture. Farmers often adjust the pH of their soil by adding lime (a base) to neutralize acidic conditions, promoting optimal plant growth. In medicine, antacids utilize neutralization to alleviate heartburn by neutralizing excess stomach acid. These examples demonstrate the practical applications of this fundamental chemical principle.
Another benefit lies in industrial processes. Many manufacturing processes require precise pH control, and neutralization reactions play a key role in achieving this balance. For example, in the production of certain pharmaceuticals, maintaining a specific pH is crucial for the stability and efficacy of the final product.
Neutralization is also critical in everyday life. From baking soda neutralizing stomach acid to cleaning products utilizing basic solutions to neutralize grease and grime, the principles of neutralization are at play in countless common scenarios.
Advantages and Disadvantages of Controlling Neutralization Reactions
| Advantages | Disadvantages |
|---|---|
| Wastewater treatment | Potential for uncontrolled reactions |
| Soil pH adjustment for agriculture | Environmental concerns related to disposal |
| Medical applications (antacids) | Cost of reagents for large-scale applications |
One real-world example of neutralization is the use of lime to neutralize acid mine drainage. Acidic wastewater from mines can contaminate surrounding ecosystems, but by adding lime, the pH can be raised, preventing environmental damage.
A frequent question is, "What are the products of a neutralization reaction?" The answer, consistently, is salt and water. This seemingly simple reaction has profound implications across numerous scientific disciplines and everyday applications.
In conclusion, the question "what does neutralization produce?" unveils a fundamental chemical process with wide-ranging implications. From environmental science to medicine, industrial processes to everyday life, the formation of salt and water through neutralization reactions plays a crucial role. Understanding the principles of neutralization empowers us to harness its transformative power, promoting sustainability, improving health, and advancing scientific knowledge. By appreciating the elegant balance achieved through this seemingly simple reaction, we gain a deeper appreciation for the intricate chemistry that shapes our world.
Decoding the exterior paint matrix where to buy your perfect hue
Unlocking the secrets to perfect pressure cooker pot roast
Decoding the board shorts mystery what to wear underneath